Notice
|
2022
11.07
Events [POSTER] SITC 2022: A novel triple action and pre-clinical safety profile of SLC-3010 predict its favorable translation in the phase I clinical study poster submission
|
|---|
|
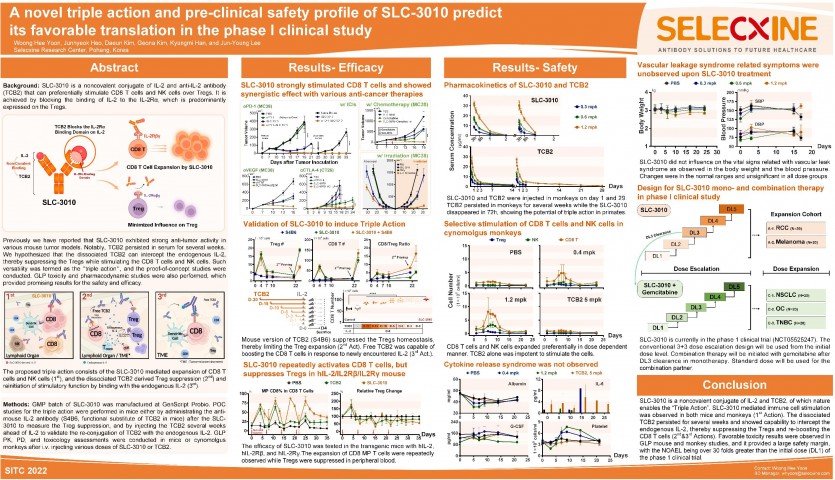
SITC 2022: SLC-3010 Poster submission Background SLC-3010 is a noncovalent conjugate of IL-2 and anti-IL-2 antibody (TCB2) that can preferentially stimulate CD8 T cells and NK cells over Tregs. It is achieved by blocking the binding of IL-2 to the IL-2Rα, which is predominantly expressed on the Tregs. Previously we have reported that SLC-3010 exhibited strong anti-tumor activity in various mouse tumor models.1 Notably, the dissociated TCB2 persisted in serum for several weeks despite the clearance of SLC-3010 within 72 h. We hypothesized that the dissociated TCB2 can intercept the endogenous IL-2, thereby suppressing the Tregs while stimulating the CD8 T cells and NK cells. Such versatility was termed as the triple action, and the proof-of-concept studies were conducted. GLP toxicity and pharmacodynamic studies were also performed, which provided promising results for the safety and efficacy. Methods GMP batch of SLC-3010 was manufactured at GenScript Probio. POC studies for the triple action were performed in mice either by administrating the anti-mouse IL-2 antibody (S4B6, functional substitute of TCB2 in mice) after the SLC-3010 to measure the Treg suppression, and by injecting the TCB2 several weeks ahead of IL-2 to validate the re-conjugation of TCB2 with the endogenous IL-2. GLP PK, PD, and toxicology assessments were conducted in mice or cynomolgus monkeys after i.v. injecting various doses of SLC-3010 or TCB2. Results The potency of the dissociated TCB2 to enable the additional anti-cancer mechanisms was studied in mice. Co-treatment of SLC-3010 and S4B6 reduced the number of Tregs and Foxp3 expression, which is a functional indicator of Treg. In addition, TCB2 remained for weeks and reinitiated the immunostimulatory function upon the IL-2 encounter. GLP PK and PD studies in the cynomolgus monkeys demonstrated the selective expansion of CD8 T cells and NK cells. Adverse effects were unobserved in all dose groups of mice and monkeys in the pathological aspects. Half-lives of SLC-3010 were approximately 14 and 8 hours in mice and monkeys, respectively. Conclusions SLC-3010 is a noncovalent conjugate of IL-2 and TCB2, of which nature enables the “triple action” including the selective stimulation of anti-cancer immunity, disruption of Treg homeostasis, and re-boosting the immune system through the conjugation with the endogenous IL-2. Favorable GLP toxicity results provided a large safety margin, with the NOAEL being over 30 folds greater than the initial dose (DL1) of the phase 1 clinical trial. |